Production Processes
We have the technological development and proven experience to carry out highly efficient production processes in each of our business lines.

[embed]https://www.youtube.com/watch?v=tikCw7VQsz4&feature=youtu.be[/embed]
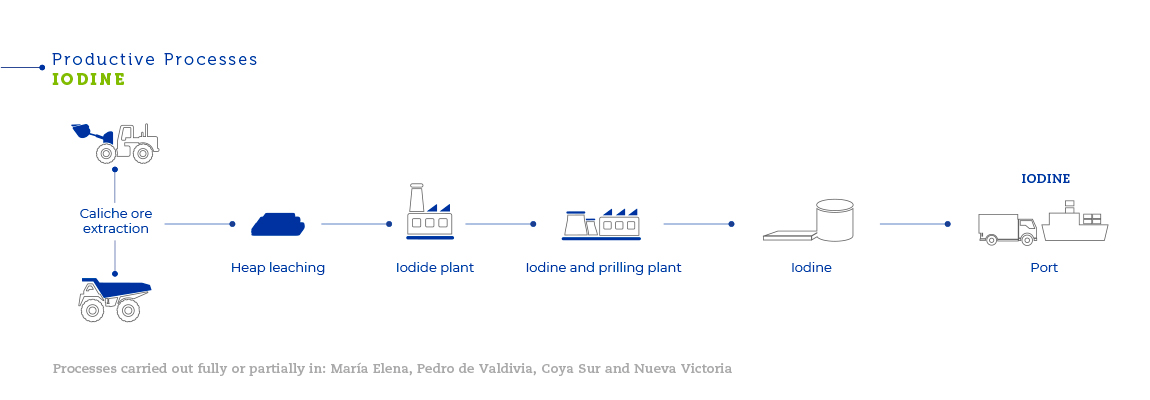
The caliche ore deposits located in northern Chile are the largest ever discovered and the only source of commercially exploitable natural nitrates in the world. Their geological origin is not clear. It is thought that the caliche ore formed from sediment deposits from an ancient inland sea or the accumulation of minerals as the result of erosion of the western face of the Andes Mountains. Caliche ore can be found beneath a blanket of inert material between 0.5 and 2.5 meters thick, in layers from 0.2 to 5 meters thick. Caliche ore concentrations vary from one mine to the next. In all, SQM extracts around 30 million tons of caliche ore each year. Caliche ore overburden is removed with bulldozers. After that, explosives are used to break the mineral into smaller pieces, which is then loaded onto trucks using front loaders. In Pedro de Valdivia, trucks carry and pile the mineral in heaps near temporary railway stations, where it is loaded onto rail cars to be taken to the plant. At María Elena, Nueva Victoria and Pampa Blanca (located in Sierra Gorda), the mineral is heap leached to obtain solutions to be used for iodine production. It is then transported to solar evaporation ponds where salts with high nitrate concentrations crystallize and are subsequently transported by truck to the Coya Sur plants to be used as an input to produce potassium nitrate. At the plants, caliche ore is mechanically ground until it reaches approximately half an inch in size. It is then transferred to a vat leaching plant where nitrate and iodine are extracted. 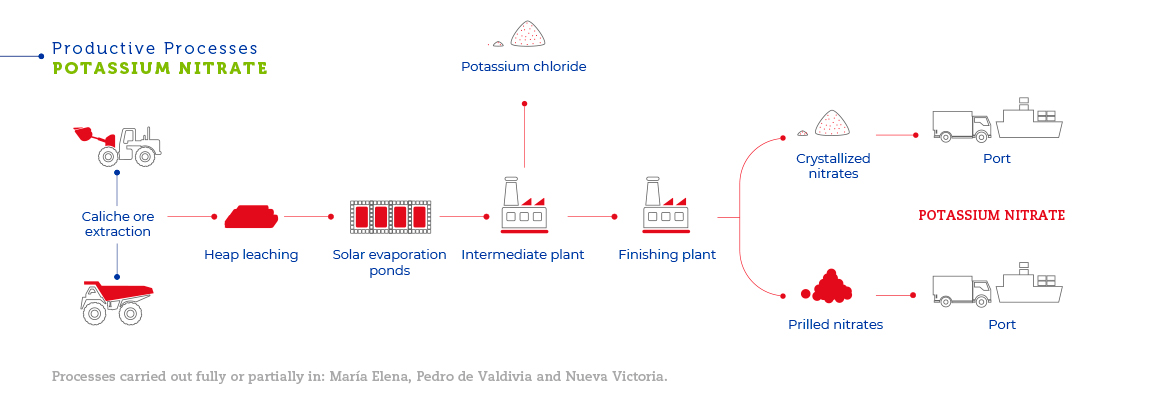

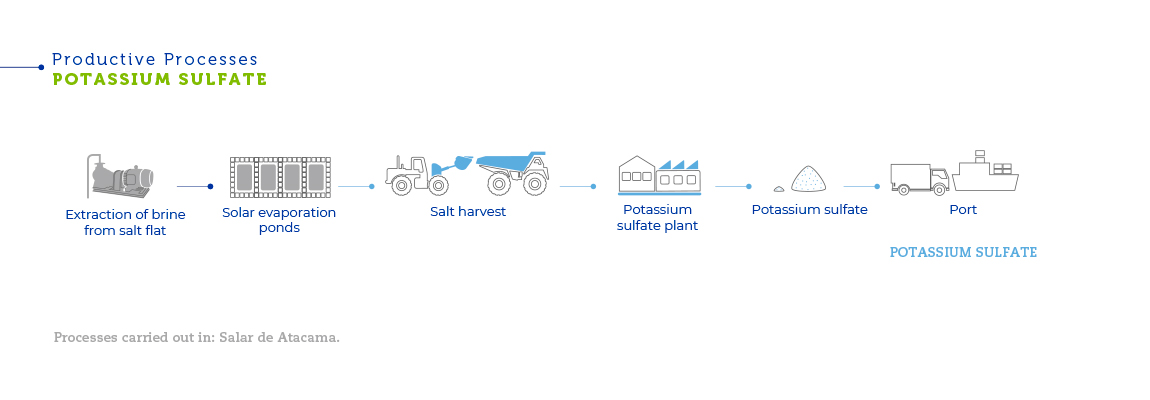
 The brines, present in pockets of liquid beneath the surface of the salt flat, are extracted with strategically placed pumping wells. After being extracted, the liquid is pumped into evaporation ponds in order to obtain concentrated brines and salts. After a series of evaporations, part of the pumped brine is recovered as a concentrated solution of lithium chloride, a byproduct of potassium chloride. That solution is transported to a plant in the Salar del Carmen, close to Antofagasta, where it is processed to obtain lithium carbonate. Another part of the already concentrated brine is reinjected into the underground deposits beneath the salt flat. The salts deposited into the evaporation ponds are then harvested and transported. After that, through grinding, flotation, drying and compacting processes, potassium chloride, potassium sulfate and boric acid are obtained.
The brines, present in pockets of liquid beneath the surface of the salt flat, are extracted with strategically placed pumping wells. After being extracted, the liquid is pumped into evaporation ponds in order to obtain concentrated brines and salts. After a series of evaporations, part of the pumped brine is recovered as a concentrated solution of lithium chloride, a byproduct of potassium chloride. That solution is transported to a plant in the Salar del Carmen, close to Antofagasta, where it is processed to obtain lithium carbonate. Another part of the already concentrated brine is reinjected into the underground deposits beneath the salt flat. The salts deposited into the evaporation ponds are then harvested and transported. After that, through grinding, flotation, drying and compacting processes, potassium chloride, potassium sulfate and boric acid are obtained. 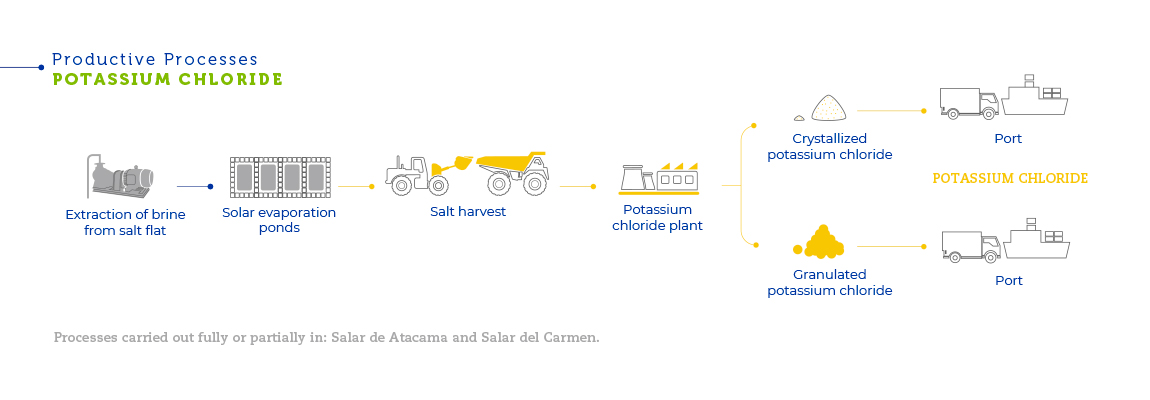
[embed]https://www.youtube.com/watch?v=g_CzEWE4y1c&feature=youtu.be[/embed]
The production of lithium carbonate and lithium hydroxide starts with the lithium chloride solutions obtained from the Salar de Atacama as a byproduct of the production of potassium chloride. These solutions are then processed to obtain lithium carbonate and lithium hydroxide at a plant in the Salar del Carmen, close to Antofagasta. The Salar de Atacama boasts the world’s largest and best lithium reserves from brines. Brines are found in the middle of the Salar de Atacama, a salty body with brine deposits generated by water filtering up from the subsoil of the Andes Mountains. 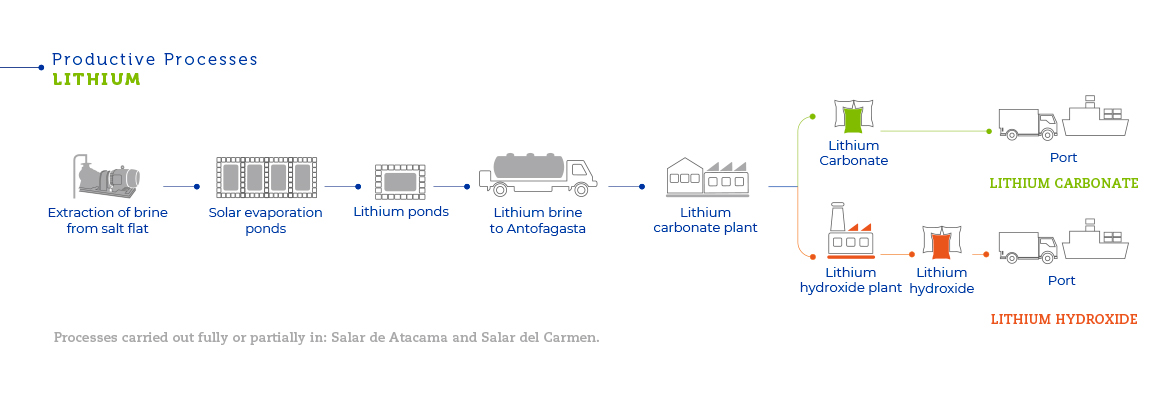

[embed]https://www.youtube.com/watch?v=g2rJiCNM2R0&feature=youtu.be[/embed]
The solutions resulting from the process of leaching caliche ore at the plants in Pedro de Valdivia, María Elena and Nueva Victoria are used to produce iodine from the iodate contained in them. Variations in iodine content and other chemical components of the treated mineral, as well as other operational parameters, require a high level of specific knowledge to efficiently manage the process. The solutions from leaching caliche ore contain iodine in the form of iodate. Part of the iodate in the solution is reduced to iodide using sulfur dioxide, which is produced by burning sulfur. The resulting iodide is combined with the rest of the original iodate solution to release the elemental iodine. Then, the solid iodine is refined using a melting and prilling process. SQM has obtained patents in Chile and the United States for its iodine prilling process.